Some Important Compounds of Transition Metals
Some Important Compounds of Transition Metals: Overview
This Topic covers sub-topics such as Potassium Permanganate, Potassium Dichromate, Uses of Potassium Permanganate, Preparation of Potassium Dichromate, Preparation of Potassium Permanganate and, Uses of Potassium Dichromate
Important Questions on Some Important Compounds of Transition Metals
On oxidation of by in neutral aqueous medium, the oxidation number of S would change from:
On complete oxidation of in acidic medium, the oxidation state of Cr will change from:
The solution of potassium dichromate is prepared from which of the following compound and how its colour changes with change in pH:
The number of moles of that will be needed to react with one mole of sulphite ion in acidic solution is:
The following steps are involved in the manufacturing of potassium dichromate:
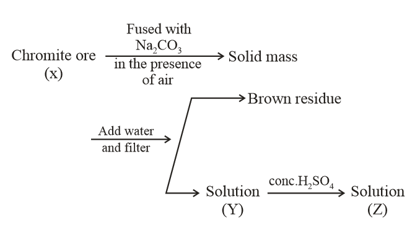
What is the difference in the oxidation number of between X and Y?
What is the number of moles of that will be needed to react with one mole of sulphite ions in acidic solution?
What would occur in the reaction between potassium permanganate, and sodium sulphite, ?
When thiosulphate ion is oxidised by iodine, which one of the following ion is produced?
When thiosulphate ion is oxidized by iodine, the new product X is formed. How many S-S linkage is/are present in X?
Balance the given chemical equation.
What is the melting point of potassium dichromate?
Potassium dichromate is a yellow crystalline solid.
_____
Which of the following species has oxygen in oxidation state?
How many peroxy linkages are present in ?
Treatment of alkaline solution with solution oxidizes iodide to:-
The above reactions give us permanganate ion from potassium oxide. The no. of manganese dioxide in the product side for both the reactions are:
Which of the following is/are correct uses of .
During oxidation of ferrous sulphate using mixture of dilute and potassium dichromate, oxidation state of chromium changes from
Write the balanced equation of the reaction of oxidising Nitrite ion to Nitrate in the presence of acidified potassium dichromate.
